The Iodine Test for Starch is used to determine the presence of starch in biological materials. The test can be qualitative or quantitative.
As a Biology Student, you will be testing for the presence of this complex carbohydrate in foods or in leaves as part of a photosynthesis experiment.These are qualitative tests.
If you study chemistry as well, you should pay attention principle of the test, as it helps explain how starch acts as an indicator for Redox Titrations or in the 'Iodine Clock Reaction'. These extensions of the Iodine Test for starch yield qualitative results.
The sole reagent required for the test is bench iodine solution (0.1 M potassium triiodide solution).
As a Biology Student, you will be testing for the presence of this complex carbohydrate in foods or in leaves as part of a photosynthesis experiment.These are qualitative tests.
If you study chemistry as well, you should pay attention principle of the test, as it helps explain how starch acts as an indicator for Redox Titrations or in the 'Iodine Clock Reaction'. These extensions of the Iodine Test for starch yield qualitative results.
The sole reagent required for the test is bench iodine solution (0.1 M potassium triiodide solution).
PROCEDURE
What is the procedure for the iodine test for starch?
The procedure for the iodine test for starch depends on whether the test sample is a solid or liquid.
SOLID SAMPLE
LIQUID SAMPLE
SOLID SAMPLE
LIQUID SAMPLE
OBSERVATIONS / RESULTS
What are the expected observations and interpretation of the iodine test for starch?
Observation
No change (Iodine remains brown) A blue-black colour develops |
Interpretation
Starch is not present Starch is present |
Are there any test samples which will not test positive for the presence of starch?
Recall that starch is a storage molecule found only in plants. Only plants and plant-based foods should test positive for the presence of starch. Any animal product testing positive for starch may be contaminated or mixed with plant product.
Note that the iodine test for starch cannot be performed on very dark solids or liquids which do not permit the observation of a colour change. Results of such an experiment are inconclusive.
Note that the iodine test for starch cannot be performed on very dark solids or liquids which do not permit the observation of a colour change. Results of such an experiment are inconclusive.
DISCUSSION: Questions & Answers
Describe the structure of starch and state which structural feature is key to the colour change in the iodine test for starch.
Starch is a polysaccharide consisting of glucose units joined together by glycosidic bonds.The chains formed during the condensation reaction are either linear or highly branched molecules.
.
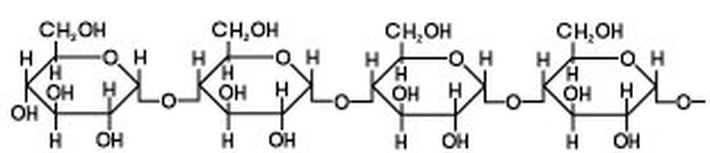
Linear - both straight and helical - molecules of starch are referred to as Amylose.
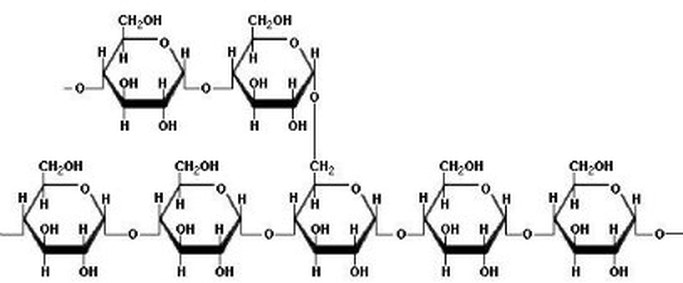
Whereas branched molecules of starch are called Amylopectin.
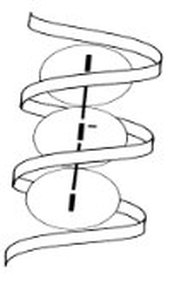
Natural starches - from plants - consist of a mixture of amylose (10 - 25%) and amylopectin (75-90%).The the structure of the helical amylose is key to the Iodine-starch reaction. A helix is a coil or a spring.
The tri-iodide and penta-iodide ions formed are linear and slip inside the helix of the amylose (form of starch).
The tri-iodide and penta-iodide ions formed are linear and slip inside the helix of the amylose (form of starch).
Describe the composition of the iodine/potassium tri-iodide reagent in the iodine test for starch.
Iodine on its own (small non-polar molecule) is insoluble in water. Therefore Potassium triiodide solution - Iodine dissolved in potassium iodide solution - is used as a reagent in the test.
To be more specific, potassium iodide dissociates, and then the Iodide ion reacts reversibly with the Iodine to yield the the triiodide ion. A further reaction between a triiodide ion and an iodine molecule yields the pentaiodide ion.
Since molecular iodine is always present in solution, the bench iodine solution appears brown; the iodide and triiodide pentaiodide ions are colourless.
To be more specific, potassium iodide dissociates, and then the Iodide ion reacts reversibly with the Iodine to yield the the triiodide ion. A further reaction between a triiodide ion and an iodine molecule yields the pentaiodide ion.
Since molecular iodine is always present in solution, the bench iodine solution appears brown; the iodide and triiodide pentaiodide ions are colourless.
Explain the principle or the basis of the colour change in the Iodine Test for Starch.
.
The starch-iodide complex is formed as charge - recall electrons are charged particles - is transferred between the starch and iodide ions - tri-iodide or pentaiodide.
The transfer of charge between the starch and the iodide ion changes the spacing between the energy levels/ orbitals.
This change results in the starch-iodide complex absorbing light at a different wavelength - than any other species aforementioned - resulting in an intense purple colour; Biologists call this colour blue-black.
The transfer of charge between the starch and the iodide ion changes the spacing between the energy levels/ orbitals.
This change results in the starch-iodide complex absorbing light at a different wavelength - than any other species aforementioned - resulting in an intense purple colour; Biologists call this colour blue-black.

Comments
Post a Comment