What is Benedict's Test for Reducing Sugars?
Benedict's Test for non-reducing Sugars is a test which determines the presence of non-reducing sugars in a test solution.
The principal reagent in Benedict's Test for Reducing Sugars is Benedict's Solution which contains
The principal reagent in Benedict's Test for Reducing Sugars is Benedict's Solution which contains
What are reducing sugars?
Sugars are classified as reducing or non-reducing based on their ability to act as a reducing agent during the Benedict's Test. A reducing agent donates electrons during a redox reaction and is itself oxidized.
The aldehyde functional group is the reducing agent in reducing sugars. Reducing sugars have either an aldehyde functional group or have a ketone group - in an open chain form - which can be converted into an aldehyde.
The aldehyde functional group is the reducing agent in reducing sugars. Reducing sugars have either an aldehyde functional group or have a ketone group - in an open chain form - which can be converted into an aldehyde.
Reducing sugars are simple sugars and include all monosaccharides and most disaccarides. Some examples of monosaccharides are glucose, fructose and galactose.Examples of reducing disaccharides are lactose and maltose.
Note that the disaccharide sucrose is not a reducing sugar. In fact, sucrose is the most common non-reducing sugar.
Note that the disaccharide sucrose is not a reducing sugar. In fact, sucrose is the most common non-reducing sugar.
Is the Benedict's Test for reducing sugars qualitative or quantitative?
The test may be qualitative, or it may be quantitative.
The qualitative test produces a colour change from blue to green to yellow to orange to brick red. The qualitative test is also regarded as semi-quantitative as the colour obtained correlates to the concentration of reducing sugars in the solution ( see observations below). This allows for a rough estimation of the amount of reducing sugar present. The qualitative test is discussed here.
The quantitative test involves the use of potassium thicyanate and the production of copper thiocyanate as white or pale green precipitate. This precipitate can then be titrated.
The qualitative test produces a colour change from blue to green to yellow to orange to brick red. The qualitative test is also regarded as semi-quantitative as the colour obtained correlates to the concentration of reducing sugars in the solution ( see observations below). This allows for a rough estimation of the amount of reducing sugar present. The qualitative test is discussed here.
The quantitative test involves the use of potassium thicyanate and the production of copper thiocyanate as white or pale green precipitate. This precipitate can then be titrated.
PROCEDURE
What is the procedure for the Benedict's Test for reducing sugars?
A liquid food sample does not need prior preparation except dilution if viscous or concentrated.
For a solid sample prepare a test solution by crushing the food and adding a moderate amount of distilled water. Decant the suspension to remove large particles. Use the decanted liquid as the test solution.
For a solid sample prepare a test solution by crushing the food and adding a moderate amount of distilled water. Decant the suspension to remove large particles. Use the decanted liquid as the test solution.
OBSERVATIONS/RESULTS
What are the expected observations for the Benedict's Test for reducing sugars?
Observations
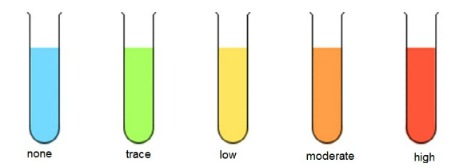
No Colour Change (Blue) Green Yellow Orange Brick-red |
Interpretations
No reducing sugars present Trace amounts of reducing sugars present Low amounts of reducing sugars present Moderate amounts of reducing sugars present Large amounts of non-reducing sugars present |
DISCUSSION
What is the principle of the Benedict's Test for reducing sugars?
Reducing Sugars have an aldehyde functional group which can reduce soluble copper (II) ions - in copper (II) sulphate - to insoluble copper (I) ions - in copper (i)oxide. The copper (I) oxide is seen as a precipitate.
State the role of copper sulphate in Benedict's Solution.
Reduced Species. The blue copper(II) ions from copper(II) sulphate are reduced to red copper(I) ions by the aldehyde groups in the reducing sugars. This accounts for the colour changes observed.
The red copper(I) oxide formed is insoluble in water and is precipitated out of solution. This accounts for the precipitate formed.
As the concentration of reducing sugar increases, the nearer the final colour is to brick-red and the greater the precipitate formed.
The red copper(I) oxide formed is insoluble in water and is precipitated out of solution. This accounts for the precipitate formed.
As the concentration of reducing sugar increases, the nearer the final colour is to brick-red and the greater the precipitate formed.
State the role of sodium carbonate in Benedict's Solution.
Alkalinization. Sodium carbonate provides the alkaline conditions which are required for the redox reaction above.
State the role of sodium citrate in Benedict's Solution.
Stabilization. Sodium citrate complexes with the copper (II) ions so that they do not deteriorate to copper(I) ions during storage.
Are there alternative tests to Benedict's Test for Reducing Sugars?
The Fehling's Test for non-reducing sugar is an alternative to the Benedict's Test. However it is less popular as it less sensitive and requires that the reagents - Fehling's solutions A and B - be kept separate until the experiment is carried out.

Comments
Post a Comment